Sodemin as distributor for Everzinc's Zinc Oxide for Pharma
Sodemin S.L. is a Spanish company established in 1991, specialized in the distribution of high-quality chemical raw materials. With more than three decades of experience, Sodemin acts as an authorized distributor in Spain and selected markets of Everzinc, one of the world’s leading producers of zinc oxide.Zinc Oxide GMP Pharma Grade distributed by Sodemin is manufactured by Everzinc and using the French process (indirect process), exclusively based on high-purity SHG zinc, in full compliance with GMP standards, and can be used as an API or as an excipient in the pharmaceutical industry. This manufacturing route ensures a homogeneous, safe and fully traceable product, suitable for the most demanding healthcare and regulated applications.
Everzinc’s Zinc Oxide GMP Pharma is covered by an official CEP Certificate and fully complies with the specifications of the European Pharmacopoeia (EP) — including the latest specific monograph for zinc oxide — as well as with the United States Pharmacopeia (USP). These certifications provide a solid foundation in terms of regulatory compliance, technical reliability and continuity of supply, key values in Sodemin’s offering.
In addition to pharmaceutical grades, Everzinc also produces a wide range of technical-grade zinc oxides intended for industrial applications such as rubber, ceramics, paints, coatings, adhesives and other technical uses. These technical zinc oxide grades, also represented by Sodemin, are presented separately on the dedicated website oxidodezinc.es, focused on industrial customers and non-pharmaceutical applications.
Thanks to this combination of commercial expertise, long-term business stability and strong industrial backing, Sodemin provides reliable solutions for the pharmaceutical, cosmetic and nutritional sectors, as well as for technical and industrial applications, adapting to different regulatory and market requirements.
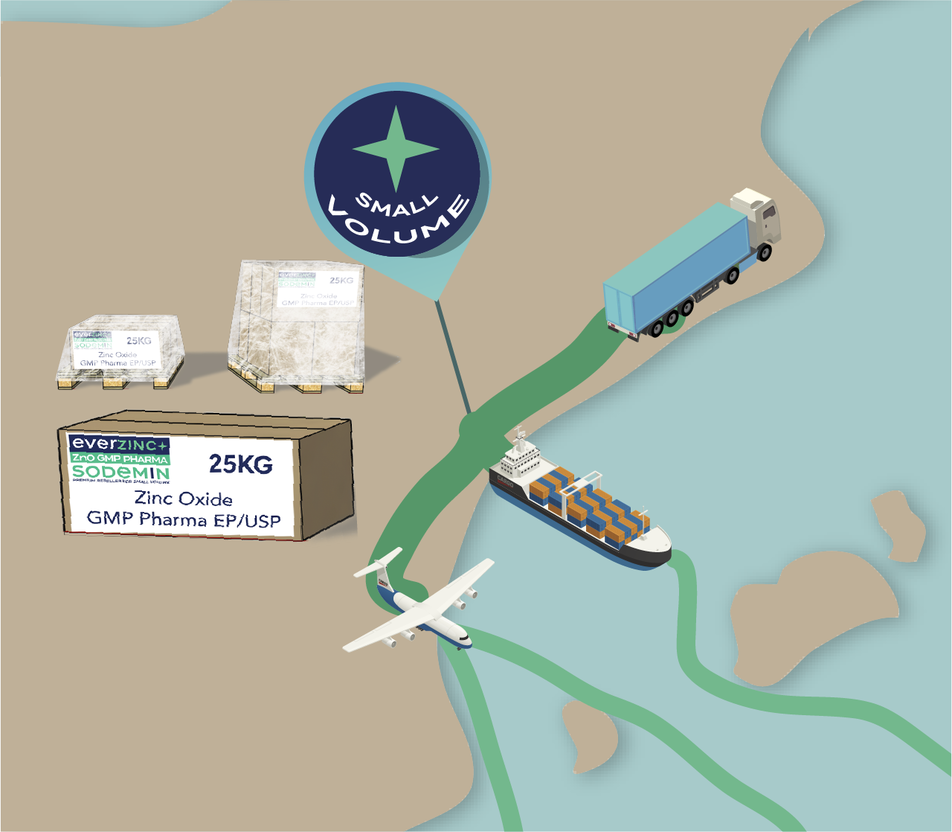
ZnO GMP Pharma EP/USP
To preserve the high quality standards of Everzinc’s Zinc Oxide GMP Pharma, Sodemin has developed a distribution and supply model aligned with GDP principles, supported by facilities operating under Sodemin’s GDP certification, and adapted to different consumption profiles and regulatory requirements.
Zinc Oxide GMP Pharma is available from minimum order sizes of 25 kg, allowing Sodemin to support specific needs, development projects or early formulation stages. At the same time, Sodemin’s logistics structure is designed to support higher-volume supply scenarios, favouring configurations that optimise operational efficiency, supply continuity and mid- to long-term planning.
All shipments are carried out in compliance with the safety regulations applicable to each transport mode — including ADR, IMDG and IATA, as applicable — in coordination with logistics partners specialised in regulated products, ensuring appropriate handling throughout all stages of transportation.
A key element of Sodemin’s approach is the coordination between supply planning and product rotation, aimed at preserving the integrity, stability and shelf life of the material. This model enables Sodemin to deliver traceable, compliant Zinc Oxide GMP Pharma under optimal conditions of use, fully aligned with the expectations of the pharmaceutical industry and regulated environments.
Contact Us
Últimas noticias
No hay noticias disponibles por el momento.





